Afrezza, a Long-Awaited Inhalable Anti-Diabetes Drug, Finally Receives U.S. FDA Approval
| Arthur Dominic Villasanta | | Jun 28, 2014 12:57 AM EDT |
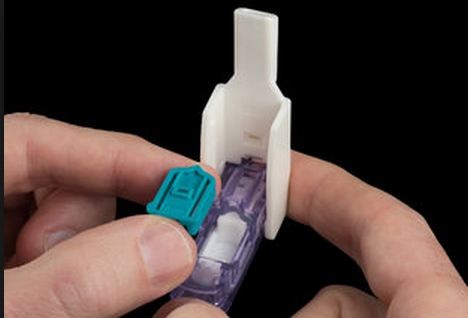
An Afrezza cartridge and its Dreamboat inhaler for diabetics
The U.S. Food and Drug Administration has approved the use of Afrezza, an inhalable, fast-acting insulin powder that comes in single-use cartridges delivered through an inhaler named Dreamboat.
Earlier, in April, the FDA's Endocrinologic and Metabolic Drugs Advisory Committee voted 13 to 1 to recommend that Afrezza (insulin human [rDNA origin]) Inhalation Powder be granted marketing approval by the FDA to improve glycemic control in adults with type 1 diabetes.
Like Us on Facebook
It later voted 14 to 0 to recommend that Afrezza be granted marketing approval by the FDA to improve glycemic control in adults with type 2 diabetes.
The FDA approval made public yesterday means Afrezza becomes the first ultra rapid-acting mealtime insulin therapy available in the U.S.
Afrezza is produced by MannKind Corporation, which is based in Valencia, California. MannKind is a biopharmaceutical company focusing on the discovery, development, and commercialization of therapeutic products for diseases such as diabetes and cancer.
The FDA cleared Afrezza for adults with the most common form of diabetes that affects over 25 million Americans. The approval decision comes more than three years after the FDA first asked MannKind to run additional clinical studies on Afrezza.
The FDA said in its approval announcement that Afrezza is not a substitute for long-acting insulin and is a new option for controlling insulin levels during meals.
It approved Afrezza with a boxed warning - the strongest type - indicating that the drug should not be used in patients with chronic lung diseases such as asthma and smoker's cough, due to reports of breathing spasms.
The FDA also requires several follow-up studies looking at the drug's long-term safety, including its impact on the heart and lungs. Mannkind first submitted the drug to FDA in March 2009.
Afrezza is an insulin powder that comes in a single-use cartridge. It is designed to be inhaled at the start of a meal or within 20 minutes.
MannKind said patients using Afrezza can achieve peak insulin levels within 12 to 15 minutes. That compares to a wait time of an hour and a half or more after patients inject insulin.
Demand for diabetes drugs is surging worldwide due to a persistent rise in obesity. The World Health Organization said 347 million people worldwide have diabetes, which is a chronic condition where the body either does not make enough insulin to break down the sugar in foods or uses insulin inefficiently.
Diabetes, which is often described as a "lifestyle disease," can lead to blindness, strokes, heart disease and death.
The FDA approval was based on a study of 3,017 patients: 1,026 with type 1 diabetes and 1,991 with type 2.
The type 1 trial showed that after 24 weeks of treatment, basal insulin plus mealtime Afrezza was noninferior to basal insulin plus insulin aspart (NovoLog) in terms of HbA1c control. The FDA noted, however, that Afrezza provided less of an HbA1c reduction than insulin aspart and the difference was statistically significant.
Patients with type 2 did better with insulin aspart and Afrezza than with a combination of insulin aspart and placebo.
TagsAfrezza, diabetes drug, inhalable, alternative
©2015 Chinatopix All rights reserved. Do not reproduce without permission
EDITOR'S PICKS
-

Did the Trump administration just announce plans for a trade war with ‘hostile’ China and Russia?
-

US Senate passes Taiwan travel bill slammed by China
-

As Yan Sihong’s family grieves, here are other Chinese students who went missing abroad. Some have never been found
-

Beijing blasts Western critics who ‘smear China’ with the term sharp power
-

China Envoy Seeks to Defuse Tensions With U.S. as a Trade War Brews
-

Singapore's Deputy PM Provides Bitcoin Vote of Confidence Amid China's Blanket Bans
-

China warns investors over risks in overseas virtual currency trading
-

Chinese government most trustworthy: survey
-

Kashima Antlers On Course For Back-To-Back Titles
MOST POPULAR
LATEST NEWS
Zhou Yongkang: China's Former Security Chief Sentenced to Life in Prison

China's former Chief of the Ministry of Public Security, Zhou Yongkang, has been given a life sentence after he was found guilty of abusing his office, bribery and deliberately ... Full Article
TRENDING STORY

China Pork Prices Expected to Stabilize As The Supplies Recover

Elephone P9000 Smartphone is now on Sale on Amazon India

There's a Big Chance Cliffhangers Won't Still Be Resolved When Grey's Anatomy Season 13 Returns

Supreme Court Ruled on Samsung vs Apple Dispute for Patent Infringement

Microsoft Surface Pro 5 Rumors and Release Date: What is the Latest?