FDA Approves Kyprolis As A Second-Line Treatment For Multiple Myeloma
| Saranya Palanisamy | | Jul 25, 2015 06:55 PM EDT |
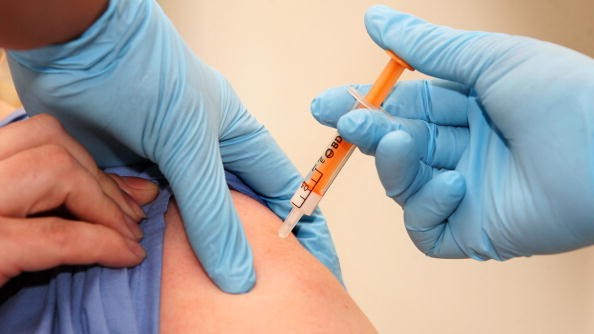
(Photo : Photo by Lewis Whyld- WPA Pool/Getty Images) Kyprolis (carfilzomib) in combination with lenalidomide and dexamethasone is approved by FDA for use in patients ailing from relapsed multiple myeloma that has received two to three prior therapies including treatment with bortezomib (Velcade, Millennium).
Kyprolis (carfilzomib) in combination with lenalidomide and dexamethasone is approved by FDA for use in patients ailing from relapsed multiple myeloma that has received two to three prior therapies including treatment with bortezomib (Velcade, Millennium).
Like Us on Facebook
Kyprolis is positioned as second line therapy for use early in the course of the treatment unlike its initial approval back in July 2012 where it is used in treating multiple myeloma as a single drug in a subset of patients, according to San Francisco Business Times.
Sean E. Harper, MD, executive vice president of R&D at Amgen, said in a press release that "The expanded indication of Kyprolis provides patients with relapsed multiple myeloma a new therapeutic option, helping to address a real unmet need for this common blood cancer." He also added that "The approval of a second indication for Kyprolis in just 3 years demonstrates that it is becoming a critical component in the treatment of multiple myeloma, and underscores our commitment to advancing care for patients with this challenging disease," reported Healio.
The FDA has given the expanded approval based on the phase 3 ASPIRE trial that is presented in the American Society of Hematology Annual Meeting in December, 2014. According to the trial results lenalidomide and dexamethasone in combination with carfilzomib increased average progression free survival of multiple myeloma patients by 8.7 months.
It is observed in further researches that the treatment worked in all the subgroups except when the patient suffered higher tumor burden at baseline. The progression free survival among patients with stage 1 disease as per international staging system (ISS) was observed to be 11 months, progression free survival of ISS stage 2 was 8 months and that of patients with ISS stage 3 was 2 months.
FDA has noted that new label on Kyprolis carries warnings on acute renal failure, hypertension, VTE, pulmonary toxicities, cardiac toxicities and warning on mortality for aged patients. It also recommends the use of carfilzomib as a monotherapy as well as modification in the dosage as either carfilzomib alone or in the combination of lenalidomide and dexamethasone, according to Haelio.
TagsKyprolis, Multiple Myeloma, Lenalidomide, Dexamethasone, Cancer
©2015 Chinatopix All rights reserved. Do not reproduce without permission
EDITOR'S PICKS
-

Did the Trump administration just announce plans for a trade war with ‘hostile’ China and Russia?
-

US Senate passes Taiwan travel bill slammed by China
-

As Yan Sihong’s family grieves, here are other Chinese students who went missing abroad. Some have never been found
-

Beijing blasts Western critics who ‘smear China’ with the term sharp power
-

China Envoy Seeks to Defuse Tensions With U.S. as a Trade War Brews
-

Singapore's Deputy PM Provides Bitcoin Vote of Confidence Amid China's Blanket Bans
-

China warns investors over risks in overseas virtual currency trading
-

Chinese government most trustworthy: survey
-

Kashima Antlers On Course For Back-To-Back Titles
MOST POPULAR
LATEST NEWS
Zhou Yongkang: China's Former Security Chief Sentenced to Life in Prison

China's former Chief of the Ministry of Public Security, Zhou Yongkang, has been given a life sentence after he was found guilty of abusing his office, bribery and deliberately ... Full Article
TRENDING STORY

China Pork Prices Expected to Stabilize As The Supplies Recover

Elephone P9000 Smartphone is now on Sale on Amazon India

There's a Big Chance Cliffhangers Won't Still Be Resolved When Grey's Anatomy Season 13 Returns

Supreme Court Ruled on Samsung vs Apple Dispute for Patent Infringement

Microsoft Surface Pro 5 Rumors and Release Date: What is the Latest?